FirePASS Science
The growing global concern for the ecology, resulting in the worldwide ban on the production of the principal halogenated fire suppressants, has stimulated extensive research of new, environmentally acceptable substances. However, it has evidently been very difficult to create a chemical agent that meets all the desirable, and often contradictory, properties.
An ideal agent naturally must be highly effective at ignition and flame suppression, yet also be environmentally friendly, stable, and non-toxic for humans during and after application.
Fire prevention and control has long dealt with the familiar fire “triangle” consisting of heat, fuel, and oxygen, all three of which are required to initiate and support combustion.
It is also well established that nitrogen, constituting 79% of atmospheric air, can significantly influence combustion. Nitrogen molecules at common flame temperatures (lower than 1100 C) do not return the absorbed thermal radiation. Rather, it is continuously removed from the combustion zone by the convection process. Because of this, an increase of Nitrogen concentration in the air causes a mass – proportional increase in the total loss of emitted thermal energy, which inhibits combustion. Furthermore, increasing the nitrogen content in the gaseous mixture affects its molecular kinetic properties, reducing the availability of oxygen molecules for combustion.
The invention of FirePASS® is based on a discovery made during research conducted in the Hypoxic Room System manufactured by Hypoxico Inc. (www.hypoxico.eu) in New York. It was discovered that the processes of ignition and combustion in a normobaric, hypoxic environment are far different from the ignition and combustion process that occurs in a hypobaric natural altitude environment with the same partial pressure of oxygen (ie up a mountain).
For example, air with a 4.51″ (114.5 mm of mercury) partial pressure of oxygen at an altitude of 9,000’ (2700 m) can easily support the burning of a candle or the ignition of paper. However, if a corresponding normobaric environment is created with the same partial pressure of oxygen (4.51” or 114.5 mm of mercury), a candle will not burn and paper will not ignite. Even a match will be instantly extinguished after the depletion of the oxygen-carrying chemicals on its tip. Consequently, any fire that is introduced into this breathable normobaric, hypoxic atmosphere is instantly extinguished. Kerosene fuel, gas lighter or propane gas torch will not ignite in this environment either.
This surprising observation leads to an obvious question: “Why do two environments which contain identical partial pressures of oxygen (ie the same number of oxygen molecules per specific volume) affect the processes of ignition and combustion so differently?”
The answer is simple: “The difference in oxygen concentration in these two environments diminishes the availability of oxygen to support combustion. This happens due to the increased number of nitrogen molecules interfering with the kinetic properties of oxygen molecules”. In other words, the increased density of nitrogen molecules in the normobaric environment creates a “buffer zone” that obstructs the availability of oxygen molecules for combustion. When the kinetic properties of both gases are compared it is revealed that nitrogen molecules are both slower and have a lower penetration rate (by a factor of 2.5) than oxygen molecules.

Figure 1 presents a schematic view of the density of oxygen and nitrogen molecules in a hypobaric or natural environment at an altitude of 9,000’ or 2.7 km. (All other atmospheric gases are disregarded in order to simplify the following explanations). Blue circles represent oxygen molecules, and green circles represent nitrogen molecules.
Figure 2 shows the density of molecules in a hypoxic environment with the same partial pressure of oxygen (4.51” or 114.5 mm of mercury), but at a standard atmospheric pressure of 760 mm of mercury. This environment contains approximately 15% of oxygen by volume, which is perfectly suitable for human life, but is not sufficient to support combustion.
As can be seen, both environments contain identical amounts of oxygen molecules per specific volume. However, the relative amount of nitrogen molecules versus oxygen molecules is approximately 6:1 in the second case (Figure 2) compare to 4:1 in the altitude air (Figure 1).

Figure 3 shows ambient air at sea level with the greater partial pressure (159.16 mm of mercury) of oxygen than in the air found at an altitude of 9,000’ or 2.7 km (114.5 mm). It should be noted that ambient air in any portion of the Earth’s atmosphere (from sea level to the top Mount Everest) has an oxygen concentration of 20.94% by volume. However, the ambient air at sea level is under a substantially higher pressure: As the number of gas molecules per specific volume increases, so the distance between the gas molecules is reduced, and the availability of oxygen to support combustion is unaffected.
Specialists of FirePASS Corporation discovered and studied the phenomenon of Ignition Suppression and Flame Extinction in the normobaric environment of breathable hypoxic (oxygen-reduced) air. Practical application of these studies resulted in developing and patenting of the Fire Prevention And Suppression System (FirePASS®).
FirePASS® satisfies all critically important properties, as required to Halon 1301 alternatives, such as:
- fire suppression efficiency;
- reignition prevention
- ozone depletion potential;
- global warming potential;
- atmospheric lifetime;
- suppressant residue level;
- electrical conductivity;
- corrosivity to metals;
- polymeric materials compatibility;
- stability under long-term storage;
- toxicity of the chemical and its combustion and decomposition products;
- speed of dispersion;
- safety and occupational health requirements.
Common flammable solid materials and liquids cannot be ignited in environments with an oxygen content lower than 16% at normal (sea level) barometric pressure. However, humans can easily tolerate an oxygen-reduced atmosphere with 12 – 16% O2 (instead of ambient 20.94% O2) without health hazard (click here for references).
In order to better illustrate the differences between the functions of the two oxygen dependent systems, the flame and the human body, we can look to the schematic diagram, the “oxygen – hemoglobin saturation curve and flame extinction curve in normobaric hypoxic environment”.
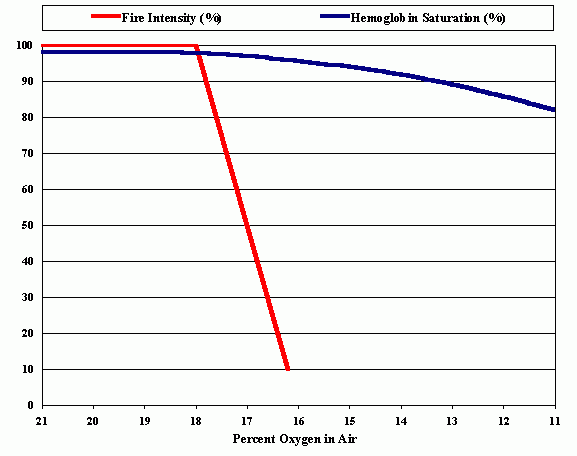
The red curve represents the decline in combustion intensity. This corresponds to the height of the stable flame and is dependent upon oxygen content in experimental environment. 100 % corresponds to the maximum flame height at ambient atmospheric oxygen content of 20.94%. Below 18% of O2 we see the continuing linear decline in height.
The blue curve shows the dependence of the oxy-hemoglobin saturation upon the partial pressure of oxygen in inspired air. Because the curve rises quickly with increase of oxygen percent, hemoglobin will be more than 90% saturated if exposed to alveolar pO2 above 60 mm.Hg (corresponds to altitude 3300 m. and 14% O2 in the normobaric hypoxic air). It should be noted that only the partial pressure of the oxygen determines hemoglobin saturation in the capillaries of alveoli. All subsequent oxygen transportation and metabolism depends exclusively upon the balance between oxygen demand and availability via the cardio-vascular system. The partial pressure of neutral diluting gases has no influence on these physiological processes at sea level conditions. In contrast, availability and reactivity of oxygen in the combustion process depends significantly upon the molecular concentrations of other, even inert, diluting gases.
The affinity of O2 to hemoglobin depends only on its partial pressure, while the kinetic of combustion depends on the proportion of oxygen in the gas mixture. (References)
Creating such a low-oxygen (hypoxic) breathable atmosphere inside a human-occupied environment will completely prevent both ignition and combustion, thus eliminating the hazard of fires even starting. On the other hand, the hypoxic air being instantly released from a pressurized container, or delivered via piping, into a normally ventilated building will any extinguish fire in the occupied confinement in a short time, simultaneously evacuating combustion gases and providing breathable atmosphere for entrapped people.
References
1 Gusstaffson C, Gennser M, Oernhagen H, Derefeldt G. Effects of normobaric hypoxic confinement on Visual and Motor Performance. Aviat. Space Environ Med. 1997 ;68 : 985-92
Methods : In 3, 11-to 14-d confinements a total of 22 subjects were exposed to different levels of normobaric hypoxia (13,14 and 15 kPa O2), with intervening periods of normoxia. In each experiment eight subjects were divided into two teams, working in 6-h shifts around the clock. Subjects performed tests of spatial orientation, visual reaction time, parallel processing and motor skills. Performance tests and questionnaires were administered once or twice in every 24-h period.
Results : All of the subjects appeared to tolerate the acute reduction in oxygen partial pressure well. In many of the tests performance improved with time as a result of learning, despite reductions in oxygen level. No reduction in performance or decrease in rate of learning was observed at any of the oxygen levels tested.
Conclusions : Oxygen levels down to 14 kPa (corresponds to 13,3 % O2 in normobaric hypoxic air) appear not to impair visual and motor performance during test.
2. Textbook of Medical Physiology (Sixth ed., Arthur C. Guyton, M.D., W.B. Saunders Company, Philadelphia. 1982).
EFFECTS OF HYPOXIA
The rate of pulmonary ventilation ordinarily does not increase significantly until one has ascended to about 8000 feet. At this height the arterial oxygen saturation has fallen to approximately 93 percent, at which level the chemoreceptors respond significantly. Above 8000 feet the chemoreceptor stimulatory mechanism progressively increases the ventilation until one reaches approximately 16,000 to 20,000 feet, at which altitude the ventilation has reached a maximum of approximately 65 percent above normal if the person is exposed only acutely to the high altitude. (But several days of exposure increases ventilation about 300 percent.).
ACCLIMATIZATION TO LOW PO2
A person remaining at high altitude [or lower oxygen levels] for days, weeks or years gradually becomes acclimatized to the low PO2 so that it causes fewer and fewer deleterious effects on the body and also so that it becomes possible for the person to work harder or to ascend to still higher altitudes…
[1] Increased Pulmonary Ventilation. On immediate exposure to low PO2, the hypoxic stimulation of the chemoreceptors increases alveolar ventilation to a maximum of about 65 percent. This is an immediate compensation for the high altitude, and it alone allows the person to rise several thousand feet higher than would be possible without the increased ventilation. Then, if the person remains at a very high altitude for several days, the ventilation gradually increases to as much as 3 to 7 times normal. The basic cause of this gradual increase is the following: The immediate 65 percent increase in pulmonary ventilation on rising to a high altitude blows off large quantities of carbon dioxide, reducing the PO2 and increasing the pH of the body fluids. Both of these changes inhibit the respiratory center and thereby oppose the stimulation by the hypoxia. However, during the ensuing two to five days, this inhibition fades away, allowing the respiratory center now to respond with full force to the chemoreceptor stimuli resulting from hypoxia, and the ventilation increases to about 3 to 7 times normal. The cause of this fading inhibition is unknown, but there is some evidence that it results from reduced CSF bicarbonate ion and perhaps also reduced brain tissue bicarbonate ion. These changes, in turn, increase the pH in the fluids surrounding the chemosensitive neurons of the respiratory center, thus increasing the activity of the center…
[2] Rise in Hemoglobin During Acclimatization. Hypoxia is the principal stimulus for causing an increase in red blood cell production. Ordinarily, in full acclimatization to low oxygen the hematocrit rises from a normal value of 40 to 45 to an average of 60 to 65, with an average increase in hemoglobin concentration from a normal of 15 gm. per cent to about 22 gm. per cent. In addition, the blood volume also increases, often by as much as 20 to 30 per cent, resulting in a total increase in circulating hemoglobin of as much as 50 to 90 per cent. Unfortunately, this increase in hemoglobin and blood volume is a slow one, having almost no effect until after two to three weeks, reaching half development in a month or so, and becoming fully developed only after many months. Decreased Affinity of the Hemoglobin for Oxygen Under Hypoxic Conditions. Within a few hours after the blood is first exposed to hypoxia at high altitudes, increased quantities of phosphate compounds are formed inside the red blood cells, and some of these combine with the hemoglobin to decrease its affinity for oxygen, that is, to shift the hemoglobin-oxygen saturation curve toward higher PO2s… One of these, 2,3-diphosphoglycerate, commonly called 2.3-DPG, is especially significant. Because of the reduced affinity for oxygen. the hemoglobin delivers the oxygen to the tissue cells at a higher PO2. At altitudes up to about 15,000 feet this effect increases oxygen delivery as much as 10 to 20 per cent. But at still higher altitudes the decreased affinity for oxygen also decreases the pickup of oxygen by the hemoglobin in the lungs, and therefore it decreases the overall availability of oxygen to tissues, which is more harmful than the increased tendency for oxygen release by the hemoglobin at tissue level is helpful.
[3] Increased Diffusing Capacity During Acclimatization. It will be recalled that the normal diffusing capacity for oxygen through the pulmonary membrane is approximately 21 mi. per mm. Hg pressure gradient per minute and this diffusing capacity can increase as much as three-fold during exercise. A similar increase in diffusing capacity occurs at high altitude. Part of the increase probably results from greatly increased pulmonary capillary blood volume, which extends the capillaries and increases the surface through which oxygen can diffuse into the blood. Another part results from an increase in lung volume, which expands the surface area of the alveolar membrane. A final part results from an increase in pulmonary arterial pressure; this forces blood into greater numbers of alveolar capillaries than normally—especially in the upper parts of the lungs, which are poorly perfused under usual conditions.
[4] The Circulatory System in Acclimatization Increased Vascularity. The cardiac output increases as much as 20 to 30 per cent immediately after a person ascends to a high altitude, but it usually falls back to normal within a few days. In the meantime, though, the blood flow through certain organs, such the skin and kidneys, decreases, while the flow through the muscles, heart, brain, and other organs that normally require large quantities of oxygen increases. Furthermore, histological studies of animals that have been exposed to low oxygen levels for months or years show greatly increased vascularity (increased numbers and sizes of capillaries) of the hypoxic tissues. This helps to explain what happens to the 20 to 30 per cent increase in blood volume and it means that the blood comes into much closer contact with the tissue cells than normally.
[5] Cellular Acclimatization. In animals native to altitudes of 13,000 to 17,000 feet, mitochondria and certain cellular oxidative enzyme systems are more plentiful than in sea level inhabitants. Therefore, it is presumed that acclimatized human beings as well as these animals can utilize oxygen more effectively than can their sea level counterparts.